Nessi, E., Lamprou, E., Stergioudi, F., Michailidis, N., Seferlis, P., Hall, J., & Papadopoulos, A. (2024). Combined Physical Property and Corrosion Assessment of Advanced Solvents for CO2 Capture Systems. 17th International Conference on Greenhouse Gas Control Technologies (GHGT-17), Calgary, Canada.
Haslam, A., Galindo, A., Adjiman, C., & Jackson, G. (2024, June). Modelling the phase behaviour of fluid systems relevant for carbon capture processes: the importance of SOx and NOx. 33rd European Symposium on Applied Thermodynamics (ESAT 2024), Edinburgh UK.
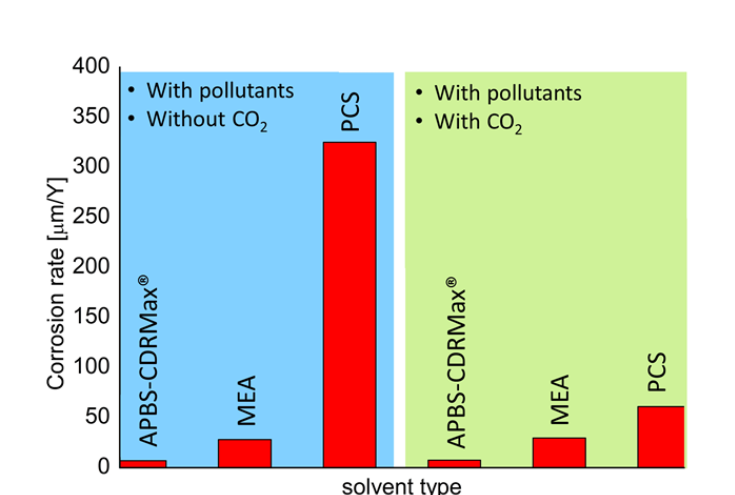
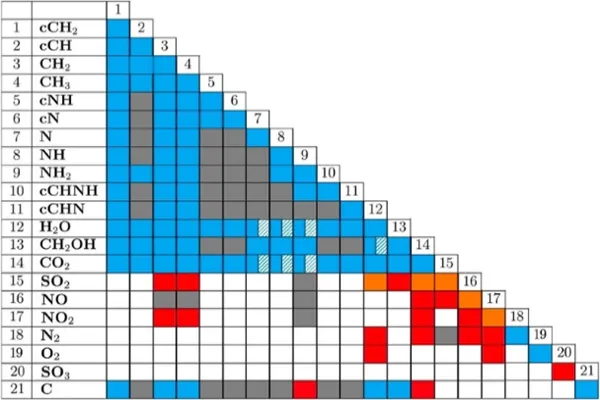
EQUILIBRIUM, KINETIC AND CORROSION TESTING WITH THE ADVANCED APBS - CDRMAX SOLVENT
Activities
Key results
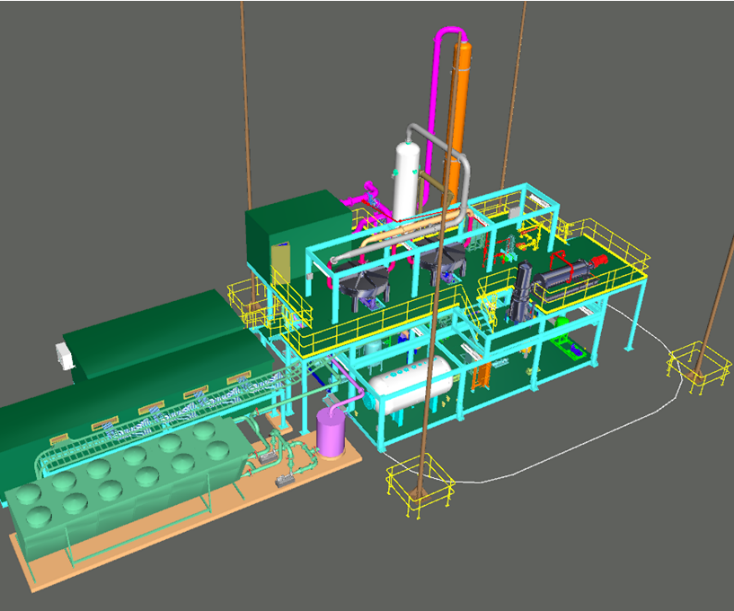
DESIGN AND CONSTRUCTION OF PILOT PLANT
Activities
Key results

DEMONSTRATION OF ADVANCED RPB CAPTURE PLANT AT 3 INDUSTRIAL SITES IN EUROPE (QUICKLIME, POWER GENERATION, REFINERY)
[1] Robinson Medici, A., Kantouros, B., Prousalis, T., de Vries, W., Seferlis, P., Papadopoulos, A., & Papadokonstantakis, S. (2025). Life-Cycle Assessment of Solvent-based Post-Combustion Carbon Capture, Storage and Utilization (CCS/CCU) using Rotating Packed Bed (RPB) reactors. Trondheim CCS Conference (TCCS-13), Trondheim, Norway.
https://doi.org/10.5281/zenodo.16406855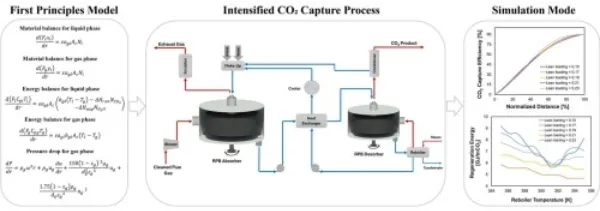
[2] Kantouros, B., Kazepidis, P., Papadopoulos, A.I., Seferlis, P., Rotating packed beds for post-combustion CO2 capture: Holistic process modeling and plant design, Chemical Engineering Science.
https://doi.org/10.1016/j.ces.2025.122466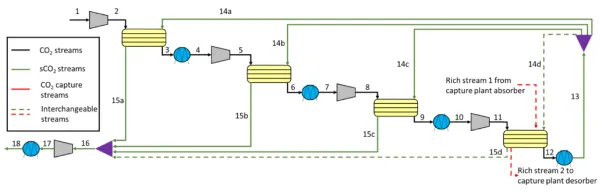
[3] Kazepidis, P., Seferlis, P., & Papadopoulos, A. (2024). Energy Recovery Strategies in CO2 Compression Using an Integrated Supercritical Rankine Cycle. Chemical Engineering Transactions, 114, 559–564.
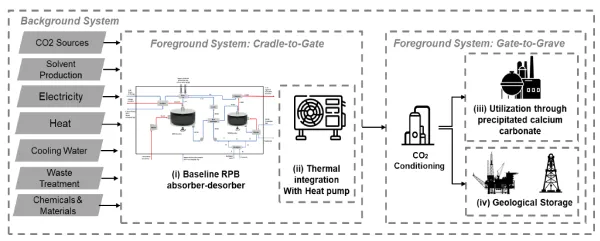
https://doi.org/10.3303/CET24114094PERFORM SCALE-UP, UTILIZATION AND SEQUESTRATION STUDIES CONSIDERING AN INDUSTRIAL CLUSTER
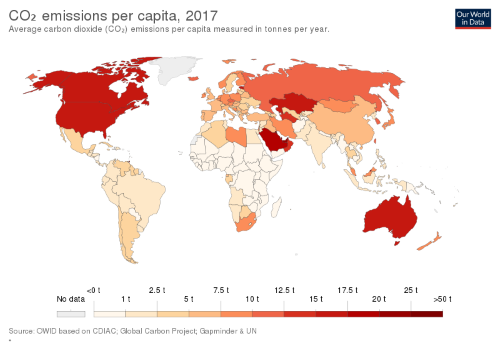
PERFORM SOCIETAL STUDIES AND POLICY ASSESSMENT TO INVESTIGATE THE EFFECTS OF THE DEVELOPED TECHNOLOGIES ON SOCIETY




